
Navigated Transcranial Magnetic Stimulation for Mapping the Motor Cortex in Patients With Rolandic Brain Tumors
Satoshi Takahashi, M.D., Ph.D., Peter Vajkoczy, M.D., Ph.D., Thomas Picht, M.D. | Neurosurg Focus. 2013;34(4):e3
Copyright © 2014 by WebMD LLC. All rights reserved.
ABSTRACT:
Object. Navigated transcranial magnetic stimulation (nTMS) is a novel technology in the field of neurosurgery for noninvasive delineation of cortical functional topography. This study addresses the spatial accuracy and clinical usefulness of nTMS in brain tumor surgery in or near the motor cortex based on a systematic review of observational studies.
Methods. A systematic search retrieved 11 reports published up to October 2012 in which adult patients were examined with nTMS prior to surgery. Quality criteria consisted of documentation of the influence of nTMS brain mapping on clinical decision making in a standardized prospective manner and/or performance of intraoperative direct electrical stimulation (DES) and comparison with nTMS results. Cross-observational assessment of nTMS accuracy was established by calculating a weighted mean distance between nTMS and DES.
Results. ll studies reviewed in this article concluded that nTMS correlated well with the “gold standard” of DES. The mean distance between motor cortex identified on nTMS and DES by using the mean distance in 81 patients described in 6 quantitatively evaluated studies was 6.18 mm. The nTMS results changed the surgical strategy based on anatomical imaging alone in 25.3% of all patients, based on the data obtained in 87 patients in 2 studies.
Conclusions. The nTMS technique spatially correlates well with the gold standard of DES. Its functional information benefits surgical decision making and changes the treatment strategy in one-fourth of cases.
Introduction
The goal of brain tumor surgery is to maximize the extent of tumor resection while preserving function. To achieve this goal is challenging, especially in glioma surgery, since these operations often involve structures that potentially carry essential function.[18] To minimize the risk for new neurological sequelae as well as the risk of leaving residual tumor, precise knowledge of the individual functional topography is indispensable. Because of natural anatomical variation between all people, displacement of familiar anatomical landmarks by the tumor, and tumor-induced plastic reorganization of functional areas, functionally relevant brain tissue cannot be reliably predicted from standard anatomical imaging alone. Thus, it is essential to obtain case-specific knowledge of the location of functionally essential brain tissue. Intraoperative brain mapping by means of DES has been so far the most accurate and reliable gold standard to obtain such knowledge. However, if we could obtain functional maps that delineate resectable versus nonresectable brain tissue outside the operating theater with the same accuracy and reliability as DES, it would be of great use.
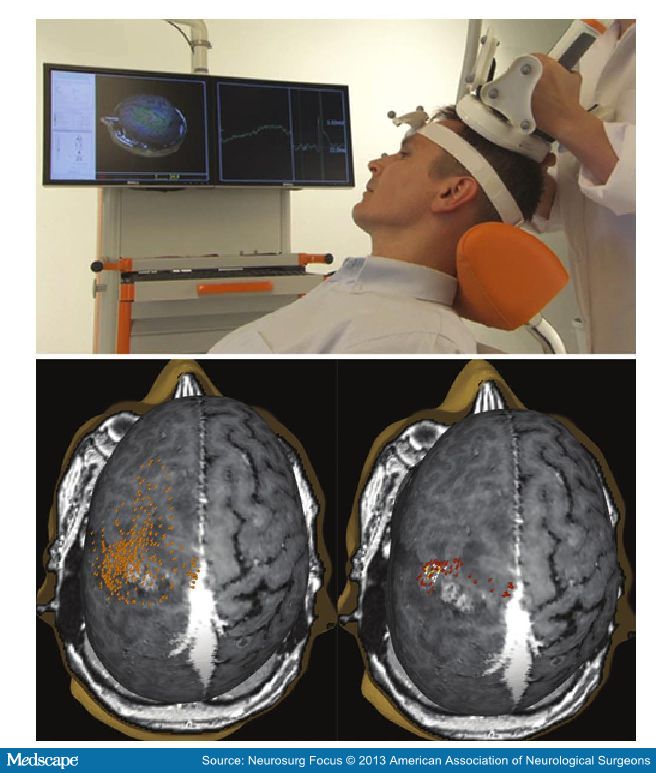
Transcranial magnetic stimulation is the only modality that is analogous to DES in that it allows for electrical stimulation of the brain and observation of the induced effect (Fig. 1). Although TMS was introduced in clinical neurology in 1985,[1] its use in neurosurgery has been sporadic, and only recently an increasing number of reports from neurosurgical institutions on the use of nTMS for brain mapping in patients with rolandic brain tumors have been published.[3,4,6,8–11,13,14,21]
Methods
To establish the clinical usability of nTMS, it must be assessed relative to DES, because DES has been the gold standard method of brain mapping for many years.
For that purpose we have conducted a review of the literature up to October 2012. In our review we have evaluated the studies that have assessed the ability of nTMS to identify the motor cortex and delineate the cortical representation of individual muscles. The search terms we used on PubMed were “transcranial magnetic stimulation,” “TMS,” “direct cortical stimulation,” “direct electrical stimulation,” “DCS,” “DES,” “motor cortex,” “M1,” and “brain tumo(u)r.” We reviewed the abstracts of those studies, and if they reported on evaluation of patients with rolandic tumors by using both nTMS and DES and/or if they assessed the impact of nTMS on surgical decision making, then we extracted information from the report
Literature Review
A total of 11 studies meeting criteria written in the Methods section were identified (Table 1). For mapping of the motor cortex, most studies used a biphasic TMS pulse (250–280 μsec pulse length) from a figure-eight coil with an outer diameter of 70 mm applied at 110% of the resting motor threshold and a maximum frequency of 0.25 Hz.[2–5,7–9,12,14–17,20,21] For lower-extremity stimulation the intensity was adapted on an individual basis.
The first study to compare spatial accuracy of nTMS to that of DES for evaluating the motor cortex was done by Krings et al.[10] They used a mechanical stereotactic arm for navigating the TMS system. Areas of motor responses identified by both nTMS and DES were compared in 2 patients with rolandic tumors. The discrepancy between nTMS and DES maps reported in the paper was never more than 1 cm. The major limitations of this study are that it reported on only 2 cases and that a homemade system that is not commercially available was used.
The next study was published more than a decade later.[13] In this study, the motor cortex of 10 patients with rolandic tumors was evaluated with a homemade nTMS system in which an electromagnetic navigation system was integrated with TMS for the purpose of positioning the TMS coil. The mean distance between hotspots of the 2 modalities was 3.4 (SD 3.0) mm (range 0–7 mm). The limitation of this study was that the pre- and intraoperative mappings were performed in the same predefined 5-mm raster, so the resulting comparative data were not fully quantitative but were semiquantitative. This system is not commercially available either, so the applicability of the findings is limited.
The next study[6] evaluated hotspots determined by nTMS and DES in 2 patients with brain tumors. In 1 patient the distance between hotspots was estimated at less than 5 mm, but in the other case the comparison referred to the distance between hotspots by preoperative nTMS and DES that was conducted after tumor removal. So the latter data are unreliable because brain shift is likely to have occurred. This study also suffers from the very small sample size.
These first 3 studies all used self-made systems, were semiquantitative, and/or had a sample size that was too small from which to draw general conclusions about the spatial accuracy of nTMS. Since then 8 more studies have been published (up to October 2012—2 of these studies[3,19] were published online first in 2012 and then appeared in print in 2013). These studies contain larger clinical samples, and all but one[3] were quantitatively evaluated. The challenge of visualizing the stimulation point at the “correct” (that is, the most likely) anatomical area where the stimulation would be effective, has been addressed differently by different systems. The first-generation nTMS systems projected the midpoint of the stimulation coil orthogonally onto the underlying cortex.[6,10,11,13] This approach ignored the significant effect that the individual cortical anatomy, the tilting angle, and the rotation of the coil have on the resulting effective “e-field” distribution. The more recent studies were conducted with a system that projects the calculated e-field induced by the magnetic pulse onto a 3D model of the brain, which is locally fitted with up to 40,000 spheres for e-field modeling. This approach assures a high accuracy in matching the virtual stimulation point to the “real” anatomy derived from the anatomical MRI scan.[3,4,8,9,14,21]
The first of the 8 studies[14] performed using a second-generation TMS system was in 20 patients with rolandic tumors, although only 17 had surgery and thus DES. In this study, DES locations were chosen independently of nTMS, and the distance between nTMS and DES hotspots was quantitatively determined. The mean (SE) distance between the nTMS and DES hotspots was 7.83 (1.18) mm for the APB muscle (n = 15) and 7.07 (0.88) mm for the TA muscle (n = 8). Importantly, the mean (SE) distance decreased to 4.70 (1.09) mm for APB (n = 8) and 5.61 (0.47) mm for TA (n = 5) after exclusion of the patients in whom possibly insufficient (< 15 stimulations) DES mapping was performed for these muscles. This study also reported comparisons on 3 other muscles in subsets of the sample. In the same year, Forster et al.[4] reported their experience with nTMS in 10 patients with rolandic tumors when compared with DES and fMRI. This study has been of particular interest because it first provided a simultaneous comparison of TMS, fMRI, and DES, thus enabling neurosurgeons to compare their options for preoperative mapping. The mean (SD) distance between the hotspots evaluated by nTMS and DES was 10.49 (5.67) mm (range 2.6–27.6 mm). One problem with this calculation, though, was that the pairs of nTMS and DES hotspots compared were from 9 different muscles. Nonetheless, this result was smaller than the mean (SD) distance between the hotspots of fMRI and DES: 15.03 (7.59) mm (range 3.4–22 mm). Therefore this study advocated that nTMS is better correlated to DES than fMRI. Yet, the use of different muscles may have accounted in part for the discrepancy between fMRI and nTMS findings: 5 hand/arm muscles, 3 leg muscles, and 1 facial muscle were recorded for TMS, whereas activation areas from the first dorsal interosseous muscle or toe movement were obtained for fMRI. One other interesting finding from this study was that the median distance for the TA muscle relative to DES was larger for nTMS (11.1 mm [range 5.9–15.9 mm]) than for fMRI (9.4 mm [range 5.7–19.1 mm]). These data suggested that nTMS may be less accurate for deeper-lying cortical regions, such as the cortical region corresponding to leg muscles. Krieg et al.[8] reported their experience in the use of nTMS presurgically for the resection of rolandic tumors. They performed preoperative nTMS in 14 patients with lesions located within or adjacent to the precentral gyrus and in 12 patients with lesions in the subcortical white matter motor tract. In the first patient group mentioned, they compared the borders between positive and negative stimulation points for nTMS and DES on axial slices by using recalibrated screenshots and BrainLAB iPlan Net Cranial 3.0.1. Although this method of comparing areas is theoretically more accurate than the hotspot method, it is very sensitive to the examination setup and is idiosyncratic. Using this method, the mean (SD) of the distance between borders for nTMS versus DES was 4.4 (3.4) mm (range 1.9–9.2 mm). These investigators also evaluated the difference between borders delineating the primary motor cortex according to blood oxygen level–dependent data on fMRI studies and the mapping area identified by nTMS. The mean (SD) between nTMS and fMRI for this method was 9.8 (8.5) mm (range 5.3–39.7 mm) for the upper extremity and 14.7 (12.4) mm (range 8.4–33.5 mm) for the lower extremity. They mentioned that their data demonstrate that nTMS correlates well with intraoperative DES, whereas nTMS and fMRI differed significantly from each other. No comparison between preoperative fMRI and DES was performed, so it remains difficult to say based on this study whether nTMS is more accurate than fMRI. This study first described the impact of preoperative brain mapping performed using nTMS on the surgery. They reported that preoperative nTMS showed a positive influence on the operative result in 5 of 14 cases, and even changed the operative strategy in 2 cases. Another study focused on patients with relatively homogeneous brain tumors (that is, only patients with low-grade glioma with a maximum diameter of 4 cm were included). Patients were assessed using a type of nTMS system that was only used in this study.[11] A "center-of-gravity" approach was used to compare the difference between nTMS and DES. In theory, this method reflects better cortical representation of each muscle than the hotspot approach and should be encouraged in the future. The authors reported a mean distance between nTMS and DES of 4.16 mm (range 2.56–5.27 mm). The major limitations of this study were its small sample size (n = 6) and inadequate explanation of the statistical methods. The next study,[16] which included 73 patients with rolandic brain tumors, is of particular interest because it widely described the influence of nTMS on surgical planning. In this study the influence of nTMS was assessed prospectively by using a categorical ranking questionnaire. The authors found the influence of nTMS on the surgical planning to be as follows: it confirmed the expected anatomy in 21.9% of patients, added knowledge that was not used in 23.3%, added awareness of high-risk areas in 27.4%, modified the approach in 16.4%, changed the planned extent of resection in 8.2%, and changed the surgical indication in 2.7%. Indeed, preoperative nTMS made an impact on 54.8% of the patients assessed, and in 27.3% of the patients nTMS even changed the surgical strategy (modified the surgical approach in 16.4%, changed the planned extent of resection in 8.2%, and changed the surgical indication in 2.7%). In the next study identified by our review, mapping was performed in 24 patients but then comparisons were made only in 5, because positive motor sites were exposed only in 5 patients with the less invasive tailored craniotomy policy these investigators used.[21] They calculated the difference between hotspots identified by nTMS and DES at 8 points in 5 patients as a median (SE) of 2.13 (0.19) mm. An interesting point in this study is that they reported that negative nTMS mapping correlates with negative DES mapping as well; in other words, DES mapping did not find any new motor sites where TMS had not. The study also included the result of a comparison between motor areas identified by nTMS and MEG. The median (SE) distance between the 2 hotspots of 46 sites in 23 patients was reported as 4.71 (1.08) mm; unfortunately a comparison of MEG to DES was not reported. Coburger et al.[3] reported their experience comparing preoperative nTMS and fMRI with intraoperative DCS in 30 patients with brain tumors in or adjacent to the primary motor cortex. From the results of comparison of their own "accuracy score," they concluded that nTMS can be used more easily preoperatively compared with fMRI, and its cortical spatial resolution is more precise than fMRI. Their study included a large number of cases, and in contrast to all other studies they used a semiquantitative classification system with grades ranging from 1 to 4, which described the spatial accuracy of nTMS in terms of identifying the M1 region. In the study, Grade 1 represents a distinct gyral localization, Grade 2 a projection over the adjacent sulcus, and Grade 3 a projection over the adjacent gyrus. Grade 4 characterizes an inability to localize the motor cortex. With this method, the accuracy was better in nTMS compared with fMRI both for upper and lower extremities. Their result suggested that nTMS represents a highly valuable supplement for preoperative brain mapping. The latest report that was found by our review is a report by Krieg et al.[9] They reported the result of brain mapping by nTMS in 8 patients with recurrent gliomas and in 23 patients with initial operations for lesions in or adjacent to motor cortex for the purpose of prospectively evaluating nTMS accuracy in recurrent gliomas. Their study is of particular interest because they were the first to show that scarring, edema, and other effects of tumor surgery do not affect nTMS accuracy. In this study, contrary to the results of the study by Forster et al.,[4] the discrepancy between nTMS and DES for the lower extremity is smaller than that between fMRI and nTMS. In summary, all studies reviewed here concluded that nTMS correlated well with the gold standard of DES.[3,4,6,8–11,13,14,21] A total of 199 attempts in 161 patients to identify the motor cortex using nTMS were described. In only 1 patient, who had an infiltrating glioma within the somatosensory cortex, could TMS not identify any motor site.[21] We have calculated the mean distance between motor cortex identified on nTMS and DES by using the mean distance described in 6 quantitatively evaluated studies.[4,8,9,11,14,21] We have then weighted the mean from each study by the number of patients that mean was derived from. In 1 study,[14] we used only the data for APB (n = 15) for simplicity. With this method, a weighted mean distance between nTMS and DES of 6.18 mm in 81 patients was calculated. Two studies[8,16] reported the impact of preoperative nTMS examination on the therapeutic decision making in a total of 87 patients. In 98.9% of cases reliable identification of functional tissue was documented after addition of nTMS. The surgical approach based on anatomical imaging alone was changed in 14 (16.1%) of 87 patients after addition of the nTMS information, the planned extent of tumor resection was increased in 6 (6.9%) of 87 patients, and the surgical indication was changed in 2 (2.3%) of 87 patients.
Discussion
Preoperative functional brain imaging is now used widely in the context of rolandic brain tumor surgeries. The most widely adopted method is fMRI, but MEG, PET, and electroencephalography have also been used for preoperative mapping.[12] These methods are all indirect. Furthermore, fMRI and PET rely on metabolic changes due to cortical activity after voluntary action, such as muscle contraction.[15] Five studies addressed the spatial accuracy of fMRI in comparison with nTMS in identifying cortical representation of motor function.[3,4,6,8,9] All but one report stated a better correlation of nTMS than fMRI when compared with DES; only in the report by Forster et al. was the median distance for the TA muscle relative to DES larger for nTMS than for fMRI. This result raises the question whether nTMS may be less accurate for deeper-lying cortical regions, such as the cortical region corresponding to leg muscles. However, on the contrary, Coburger et al.[3] reported (using their own accuracy scores) that nTMS showed statistically significantly higher spatial accuracy of the motor representation of the lower extremity than fMRI, and Krieg et al.[8] also reported better spatial accuracy of nTMS for lower extremity over fMRI. Three studies reported hotspot distances between TMS and DCS separately for upper and lower extremities.[4,11,14] In 24 comparisons the mean distance for upper extremity was 7.73 mm (range 0.42–27.6 mm) and in 17 comparisons for lower extremity it was 9.47 mm (range 3.2–16.4 mm). Whether nTMS can identify the motor representation of the lower extremity with sufficient accuracy is currently still controversial and has to be addressed by future studies.
The spatial accuracy of nTMS for the mapping of hand and lower-arm muscles can be considered acceptable for surgical planning, as shown in the available reports.[3,4,6,8–11,13,14,21] It enables the surgeon to identify spatially accurate cortical representations of individual muscles vis-à-vis the gold standard of DES. Nevertheless, it has to be emphasized that nTMS is not a substitute for DES. Navigated TMS is performed outside the operating theater before and/or after operation, whereas DES is performed in the operating theater. This fact implies the following limitations and unique capabilities of each method: DES is an invasive method and its extent in area and time is limited by the surgical requirements. On the other hand, DES is the only modality that allows for direct identification of subcortical pathways and for monitoring of brain function at the time of surgery. The nTMS method provides information complementary to DES derived from its unique features. The overarching strength of nTMS is that it is so far the only mapping modality other than DES that is capable of stimulating brain and recording the output.
Navigated TMS can be performed repetitively preoperatively and postoperatively, and according to the literature it has the following unique capabilities that supplement the information provided by intraoperative DES. 1) The nTMS method provides an objective assessment of the possibility of recovery of motor function. For example, in patients who have become plegic, nTMS can show if motor function is still possible.[15] 2) The nTMS method provides preoperative clarification of detailed cortical motor representation, which can result in smaller craniotomies that can prevent injury to eloquent tissue; for example, surgeons can apply keyhole approaches without exposing motor cortex. According to the results of 2 prospective studies, the surgical approach was changed in 14 (16.1%) of 87 patients after nTMS mapping data were reviewed.[8,16,17] 3) The nTMS assessment can also be performed repeatedly across time, and this can enable visualization of plastic changes, which may influence the timing of the next surgical interventions; for example, eloquent tissue infiltrated by brain tumor that prevented total resection can become resectable due to shifting of the functional area over time.[20] 4) The nTMS assessment enables an objective preoperative estimation of the extent of safe cortical tumor resection. According to the results of 2 prospective studies, preoperative brain mapping performed using nTMS led to a change of planned extent of tumor resection in 6.9% of the cases, and the surgical indication was changed in 2 (2.3%) of 87 patients.[8,17] 5) The nTMS method can be used to define accurate “seed-points” for diffusion tensor imaging, to visualize the pathways of the pyramidal fiber tracts. This approach can improve the accuracy of diffusion tensor imaging.[5,7] 6) The nTMS method is applicable also in small children;[2] on the other hand, the modalities that require a patient’s voluntary movement such as fMRI sometimes cannot be performed in small children or uncooperative or disabled patients. 7) Use of nTMS enables the surgeon to assess the functional status of the motor system in patients with brain tumor. For example, a high interhemispheric ratio of resting motor threshold or a low interhemispheric ratio of MEP amplitude was reported to suggest imminent deterioration of the patient’s motor status.[17] Altogether, these 7 unique capabilities of nTMS enable neurosurgeons to improve rolandic tumor resection through advanced planning and better postoperative evaluation of the surgery.
Conclusions
Navigated transcranial magnetic stimulation is the only mapping modality so far that is capable of stimulating the brain and recording the output in a noninvasive and painless way. Its accuracy for delineation of the motor cortex is comparable to that of DES. However, DES cannot be replaced by a noninvasive method due to its unique capability to stimulate subcortical structures accurately and to monitor function during surgery. The nTMS method is clinically beneficial not only by providing the same cortical spatial information as DES outside the operating theater, but in addition due to its capacity to provide information on the functional status of the motor system, to provide prognostic information with respect to functional outcome, to be applicable in plegic patients and young children, and to identify plastic changes of functional topography. Currently the application of nTMS for analysis of language-related areas is under intense investigation, with promising results.[19]
