
rTMS Induced Tinnitus Relief Is Related to an Increase in Auditory Cortical Alpha Activity
Nadia Müller, Isabel Lorenz, Berthold Langguth, Nathan Weisz | Published: February 04, 2013 | DOI: 10.1371/journal.pone.0055557
Copyright © 2013 Plos.org All rights reserved.
Abstract
Chronic tinnitus, the continuous perception of a phantom sound, is a highly prevalent audiological symptom. A promising approach for the treatment of tinnitus is repetitive transcranial magnetic stimulation (rTMS) as this directly affects tinnitus-related brain activity. Several studies indeed show tinnitus relief after rTMS, however effects are moderate and vary strongly across patients. This may be due to a lack of knowledge regarding how rTMS affects oscillatory activity in tinnitus sufferers and which modulations are associated with tinnitus relief. In the present study we examined the effects of five different stimulation protocols (including sham) by measuring tinnitus loudness and tinnitus-related brain activity with Magnetoencephalography before and after rTMS. Changes in oscillatory activity were analysed for the stimulated auditory cortex as well as for the entire brain regarding certain frequency bands of interest (delta, theta, alpha, gamma). In line with the literature the effects of rTMS on tinnitus loudness varied strongly across patients. This variability was also reflected in the rTMS effects on oscillatory activity. Importantly, strong reductions in tinnitus loudness were associated with increases in alpha power in the stimulated auditory cortex, while an unspecific decrease in gamma and alpha power, particularly in left frontal regions, was linked to an increase in tinnitus loudness. The identification of alpha power increase as main correlate for tinnitus reduction sheds further light on the pathophysiology of tinnitus. This will hopefully stimulate the development of more effective therapy approaches.
Figures 1,2,3
Figure 1
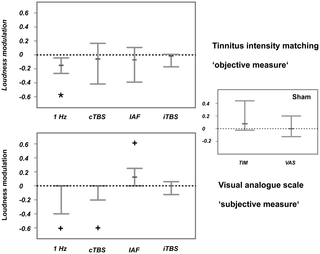
Figure 2
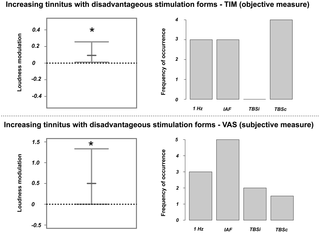
Figure 3
Citation: Müller N, Lorenz I, Langguth B, Weisz N (2013) rTMS Induced Tinnitus Relief Is Related to an Increase in Auditory Cortical Alpha Activity. PLoS ONE 8(2): e55557. doi:10.1371/journal.pone.0055557
Editor: André Mouraux, Université catholique de Louvain, Belgium
Received: September 18, 2012; Accepted: December 27, 2012; Published: February 4, 2013
Copyright: © 2013 Müller et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was supported by a grant from the Deutsche Forschungsgemeinschaft (grant number: 4156/2-1) and the Tinnitus Research Initiative. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have read the journal’s policy and have no conflicts of interest.
Introduction
Tinnitus is defined as the subjective perception of a sound in the absence of any physical sound source. If persisting longer than a certain amount of time, conventionally between six and twelve months, it is usually regarded as ‘chronic’, reflecting clinical experience that the phantom sound will persist. Chronic tinnitus is a common phenomenon with a prevalence of 5–15% of the population in western societies [1], [2]. In 1–3% of the population, tinnitus is associated with severe distress including psychiatric problems (e.g., depression), sleep disturbances, concentration problems or work impairment [1]. To date, there is no effective treatment that reliably eliminates tinnitus [1], partly because the processes that generate and maintain tinnitus and its associated problems are not completely understood. A broad consensus, however, is that tinnitus is generated in central brain structures rather than in the peripheral auditory system. Evidence comes from clinical studies showing that the tinnitus percept persists even after transection of the auditory nerve fibres [3], [4].
In most cases, tinnitus is associated with a damage of hair cells in the inner ear [5], [6], resulting in pathological neuronal activity in central structures [7]–[10]. Various neurophysiological processes at different levels of the auditory system that are elicited by hearing loss have been suggested as being involved in the generation of tinnitus [9]. Hearing loss, for instance, results in a loss of inhibition and a reorganisation of the tonotopic map [11], [1]. Studies in animals and humans demonstrate that the tinnitus sensation is associated with hyperactivity in subcortical and cortical auditory brain structures. This hyperactivity is reflected in an enhanced spontaneous firing rate [12]–[14], elevated bursting activity [13], [15] and increases in neural synchrony that have been shown to correspond closely to hearing loss [16]. Roberts et al. (2010) postulate that, among these processes, the increase in neural synchrony seems to be most relevant for the actual generation of tinnitus as it has the potential to impact postsynaptic targets and recruit cortical and downstream neurons into a tinnitus percept. The role of altered synchrony in tinnitus is strongly supported by studies that report changes in oscillatory brain activity associated with tinnitus [7], [17]–[19] on a subcortical and cortical level. On a subcortical level, for instance, abnormal low-frequency activity in the thalamus can lead to disturbances in the thalamo–cortico–thalamic network (thalamocortical dysrhythmia, TCD) and thereby influence perception [20]. On the cortical level it has been shown that oscillatory activity in the so-called alpha band (8–12 Hz), which has been related to inhibitory processes [21], is reduced in the auditory cortex of tinnitus patients [10]. Power increases were found for low frequencies in the delta [10] to theta range [20], [22], [23], [24] and in gamma power compared to normal hearing controls [7], [18], [25].
A promising treatment approach for chronic tinnitus is transcranial magnetic stimulation (TMS) [26], [27] as this affects brain activity directly, thereby holding the potential to influence abnormal ongoing brain activity related to tinnitus. Particularly in its repetitive form (rTMS; [28], [29]), it has been shown (mostly in the motor system) that stimulation-induced changes of excitability and plasticity outlast the period of stimulation. A growing number of studies indeed point to tinnitus relief after a series of ten rTMS sessions (for an overview see [30]), with effects lasting for up to four years [31]. However, the effects show great interindividual variability [27], [32] and only moderate effect sizes [33]. Only few studies have investigated the effects of rTMS on auditory cortical activity in tinnitus sufferers and which aspects of these modulations are relevant for tinnitus relief. In a previous study, we were able to demonstrate that various forms of single session rTMS (particularly cTBS, iTBS and 1 Hz rTMS) could reduce the auditory Steady State Response (aSSR), which is in turn correlated with subjectively perceived tinnitus loudness [34]. In the context of the same study we also collected resting MEG activity. To date, no published reports have investigated the impact of rTMS on spontaneous oscillatory brain activity in tinnitus patients – a potentially fundamental element in the generation of tinnitus [9], [10]. In order to better understand the pathophysiology of tinnitus and also to systematically advance tinnitus therapies, it is essential to know if and how oscillatory brain activity in tinnitus patients is modulated by rTMS and what changes in oscillatory activity are crucial for tinnitus suppression. This view is also strongly supported by Fuggetta and colleagues who emphasized that we will gain further insight into therapy approaches and the pathophysiology of a variety of neurological disorders by investigating rTMS effects on TCD-like EEG patterns [23]. With the current data we are able to show – on the level of group as well as single subject statistics – that tinnitus relief after rTMS is associated with an increase in alpha activity in the auditory cortex, thus supporting the relevance of alpha activity for tinnitus [10].
Methods
1. Participants
Ten patients with chronic tinnitus participated in the current study (7 males, 3 females). The mean age of the participants was 49.8 years (range: 21–70). Patients were recruited via advertisements in the local newspaper and flyers posted at the University of Konstanz. Tinnitus severity was assessed with Hallam’s [35] Tinnitus Questionnaire (Tinnitus-Fragebogen; [36]), revealing a mean score of 29.9 (range: 8–59). Half of the patients reported unilateral tinnitus (4 with left-sided tinnitus, 1 with right-sided tinnitus), while the other half indicated having perceived the tinnitus bilaterally. We only included patients with a maximum tinnitus duration of four years, as the impact of rTMS on chronic tinnitus declines with longer tinnitus duration [26]. All patients were informed about the content of the study as well as the potential risk factors and underwent a thorough anamnesis concerning potential contraindications for TMS (previous personal or family history of epileptic seizures, cardiac pacemaker, pregnancy, neurodegenerative diseases, brain injuries). Furthermore, patients with psychiatric or neurological disorders according to the M.I.N.I. (Mini International Neuropsychiatric Interview, German Version 5.0.0) and with anticonvulsant or tranquilizer medication were excluded from the study. The Ethics Committee of the University of Konstanz approved the experimental procedure and the participants gave their written informed consent prior to taking part in the study.
2. Experimental Design
Patients underwent five sessions of rTMS, including the measurement of tinnitus loudness and brain activity with MEG before and after rTMS, resulting in a dataset of 100 MEG and 100 tinnitus loudness measurements (10×5×2). In the five sessions, five different rTMS protocols were applied, with a minimum interval of one week between sessions and using a randomized, single-blind, placebo-controlled design. For an overview of the study design, see Figure 1.
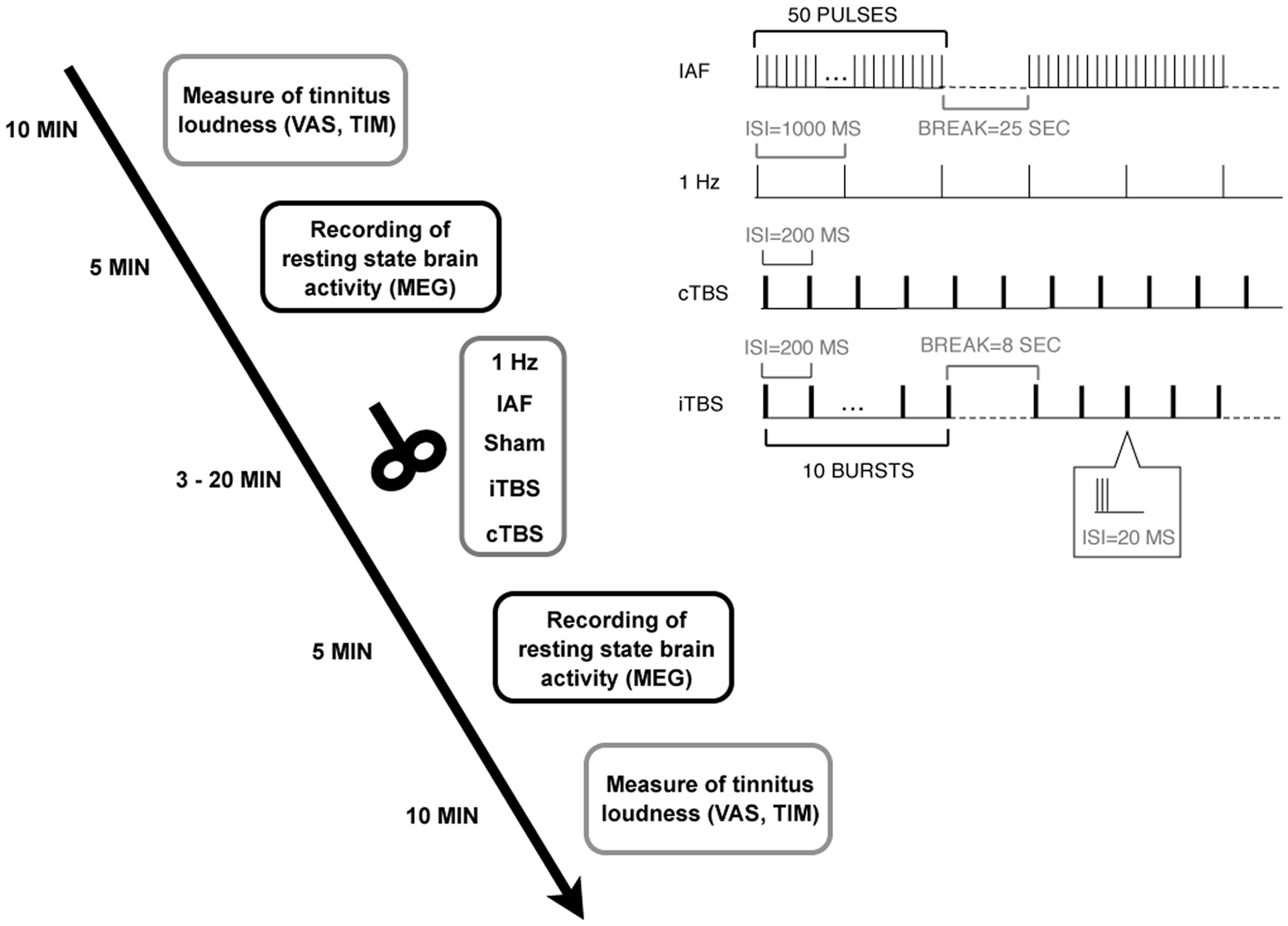
Figure 1. Experimental design.
Patients underwent five sessions with five different rTMS protocols (including Sham). In each session, tinnitus loudness and oscillatory brain activity were measured before and after rTMS. The right upper panel illustrates the different stimulation protocols.
doi:10.1371/journal.pone.0055557.g001
3. Measurement of Tinnitus Loudness
Before the first and after the second MEG recording, patients were asked to match the loudness of their tinnitus to a reference tone of 1 kHz (tinnitus intensity matching; TIM). This procedure considers the absolute hearing threshold of the 1-kHz tone so that the matched tinnitus intensity is expressed in ‘sensation level’. Additional to this psychoacoustic assessment, the patients estimated their perceived tinnitus loudness on a visual analogue scale (VAS) ranging from 0 (‘not loud at all’) to 10 (‘extremely loud’).
4. Data Acquisition with MEG
The MEG recordings were carried out using a 148-channel whole-head magnetometer system (MAGNESTM 2500 WH, 4D Neuroimaging, San Diego, USA) installed in a magnetically shielded chamber (Vakuumschmelze Hanau). Prior to the recording, individual head shapes were collected using a digitiser. Participants lay in a supine position and were asked to keep their eyes open and to focus on a fixed point on the ceiling during the recording. The recording time was five minutes. MEG signals were recorded with a sampling rate of 2034.51 Hz and a hard-wired high-pass filter of 0.1 Hz. MEG measurements were conducted before and after TMS. The time interval between the end of the TMS session and the start of the second MEG recording did not exceed five minutes.
5. Brain Stimulation with TMS
TMS stimulation (biphasic magnetic pulses) was administered with a figure-of-eight coil (coil winding diameter 2×75 mm; Magnetic Coil Transducer C-B60, Medtronic) connected to a MagPro X 100 TMS stimulator (Medtronic A/S, Skovlunde, Denmark).
Five different stimulation protocols were applied in randomized order over five sessions separated by at least one week: 1-Hz rTMS (1 train with 1000 pulses, frequency 1 Hz), individual alpha frequency rTMS (IAF; 20 trains with 50 pulses and 25 seconds inter-train interval, peak frequency ranging between 8 and 12 Hz), continuous theta burst stimulation (cTBS; 200 bursts at a frequency of 5 Hz with bursts consisting of 3 pulses at 50 Hz), intermittent theta burst stimulation (iTBS; 10 trains of 10 bursts at a frequency of 5 Hz with bursts consisting of 3 pulses at 50 Hz and an 8 seconds inter-train interval), and a placebo sham stimulation (45° coil angulation, applying the IAF protocol). Individual alpha frequency was defined as the peak of the power spectrum (between 8 and 12 Hz) at temporal sensors in the first MEG recording. For an illustration of the different protocols see right upper panel of Figure 1. The patients were blind to the TMS condition. The intensity of the stimulation was adjusted according to the resting motor threshold (RMT) –a common procedure in TMS studies [37]. RMT was measured by delivering single pulses at the optimal site over the motor cortex and defined as the lowest stimulation intensity required for producing visible hand muscle contractions in at least five out of ten trials as it has been done in previous studies [37]. For 1-Hz rTMS, IAF, and sham stimulation, intensity was set to 110% of the RMT and for iTBS and cTBS to 80% of the RMT. Thus, the intensities we applied were slightly higher than those used by Huang et al. [87] for motor cortex stimulation. To prevent hearing damage caused by the loud clicking sound of the TMS device, patients were required to use earplugs. Patients were seated in a comfortable chair while the TMS coil was fixated with a mechanical arm. The handle of the coil always pointed upwards. In case of right-ear or bilateral tinnitus, the coil was placed over left Heschl’s gyrus by moving 2.5 cm upwards from T3 on the line between T3 and Cz and then 1.5 cm perpendicularly in a posterior direction, analogously over right Heschl’s Gyrus in case of left-ear tinnitus. This procedure has been proven to reliably position the TMS coil over the auditory cortex [38].
6. Data Analysis
6.1. Preprocessing.
We analysed the data sets using Matlab (The MathWorks, Natick, MA, Version 7.5.0 R 2007b) and the Fieldtrip toolbox [39]. We separately extracted two-second epochs from the continuously recorded MEG signal for the measurements, resulting in 150 trials for the pre (baseline) and post-TMS condition, respectively. We then performed artefact rejection in two steps. First, we visually inspected trials for eye movements, muscle artefacts or channel jumps and rejected the affected trials. Furthermore, we eliminated dead and very noisy channels. Two out of 100 data sets (one from the cTBS and one from the IAF protocol) had to be excluded because of very poor data quality. In a second step, the data sets were processed using an Independent Component Analysis (ICA; http://sccn.ucsd.edu/eeglab/) to correct for heartbeat-related artefacts. We entered 80 randomly sampled trials into the ICA in order to get independent components with a distinct time course and spatial topography. We identified those components (two in the majority of cases) that captured cardiac activity through visual inspection. Afterwards, the respective weights of the ICA were applied to the whole data set, artefact components were removed and the original data were reconstructed without the impact of the artefact. To ensure similar signal-to-noise-ratio for direct comparisons between the placebo (sham) and active TMS conditions, the trial number was adjusted to the minimum remaining trial number for the two time points (pre and post) and the compared conditions (sham and the respective active TMS protocols). To keep trial numbers in a comparable range, maximum trial number was set to 90.
6.2. Spectral power analyses derived from auditory cortex.
As patients had to leave the MEG between pre and post-TMS sessions, all analyses were performed at source level in order to obtain robust effects, in contrast to a potential analysis at sensor level, which would have been more susceptible to altered head positions in the sensor helmet (from pre to post as well as over different days).
For each patient, we created a head model fitted to the head shape of the first MEG measurement using a multisphere approach [40]. This yielded a grid covering the entire brain with a resolution of 1 cm and assured that the same grid would be used in a single participant across all sessions. The leadfield for each grid point, however, was separately calculated for each session to account for potentially altered positioning of the sources with respect to the sensors.
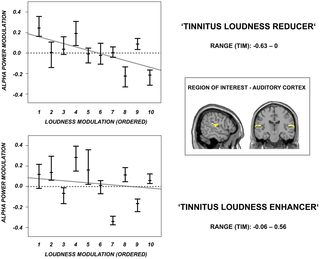
Data were then analysed for the region of interest, defined as the auditory cortex (Brodman Area 41 & 42; Talairach atlas) ipsilateral to the TMS stimulation side. We also investigated oscillatory brain activity contralateral to the stimulation side. This analysis did not reveal any consistent effects; we thus do not describe them in further detail. In order to estimate power spectra for the region of interest, we employed a multitaper spectral estimation method [41] to the ICA-corrected raw data and kept the complex Fourier coefficients. We used a different smoothing for low (2–12 Hz) and high frequencies (30–90 Hz) so that the data were multiplied with a set of orthogonal Slepian tapers, yielding a frequency smoothing of +/−1 Hz for low and +/−5 Hz for high frequencies. We then constructed spatial filters (with fixed orientation) using the lcmv-algorithm (lcmv beamformer; [42]) for each grid point within the region of interest. This was again accomplished for low and high frequencies respectively by filtering the non-ICA corrected data in the corresponding frequency bands (2–12 Hz, 30–90 Hz). Afterwards, we projected the complex values into source space by multiplying them with the accordant spatial filters and by calculating the complex modulus of the values. We thereby obtained absolute power values for each voxel within the region of interest. By averaging the values in the region of interest we obtained one single value for each frequency. This procedure was repeated for each patient, for both time points (pre and post) and for the five different conditions (4 active TMS protocols & sham). Finally, spectral source estimates were normalized for each patient and condition by calculating a (post-pre)/pre ratio, reflecting the modulation of oscillatory power from pre to post TMS intervention. It should be noted that we focused on frequencies of interest that were derived from previous studies on altered auditory oscillatory power in tinnitus patients: delta (1–3 Hz; [10]), theta (4–6 Hz; [20], [22]; alpha (8–12 Hz; [10]), gamma ([7], [18], [25]) subdivided in low gamma (30–70 Hz) and high gamma (70–90 Hz). In the next step, these values were statistically tested.
6.3. Statistical analyses of the pre-post modulations.
Statistical analyses were performed using R version 2.11.1 for Mac OS X (www.r.project.org). As the complex study design entailed a small sample size and we identified various ‘outliers’ that, after precise investigation, we did not wish to treat as real outliers, we used a bootstrap approach for the statistical analysis. The ‘outliers’ or extreme values were not due to poor data quality, but rather reflect strong interindividual differences in the different stimulation protocols with somehow systematic patterns (only for specific TMS protocols and in patients with very short tinnitus duration; see Figure S1 in the supplemental material for comparison). Therefore, we decided not to exclude these cases and instead use robust statistics. We always compared the pre-post ratios of the active TMS conditions against sham using a bootstrap approach (‘boot’ package included in R). Thus, the sham values were subtracted from the activation values for each patient. After that we created 1000 new samples from the original sample (by drawing with replacement). For each of these new samples (having the same size as the original sample) the median was calculated. We thereby obtained a distribution of 1000 bootstrapped medians. The median was used in order to not overemphasize extreme values. We subsequently extracted the 95% confidence intervals (CI) for the median for each stimulation form respectively. If the confidence interval did not include 0, the effect could be considered as significant (power modulation significantly different in the active TMS vs sham TMS condition).
We performed this procedure for both, the modulation of tinnitus loudness and the modulation of auditory oscillatory activity in the frequency bands of interest.
6.4. Signatures of auditory brain activity reducing tinnitus loudness.
Apart from analyses that focused on consistent modulations of tinnitus loudness and oscillatory activity after the different TMS protocols, we wanted to identify the signatures of oscillatory brain activity that are decisive for a strong reduction or an enhancement of tinnitus loudness. Hence, we defined the most effective stimulation protocol (among active TMS conditions) in reducing tinnitus loudness according to the TIM scores for each patient and analysed the according modulations in oscillatory activity. We repeated this procedure for the stimulation protocols that increased tinnitus loudness. For the selection of the according TMS protocol we used the TIM scores as they clearly separated the different protocols, whereas the VAS scores were more ambiguous (i.e., different protocols lead to identical modulations of tinnitus loudness). Note that we obtained similar results when excluding the ambiguous cases in the VAS assignment compared to the TIM assignment.
We conducted a further bootstrap statistic (the same method as described above) for the five frequency bands of interest (delta, theta, alpha, low gamma, high gamma) and for both increasing and decreasing tinnitus loudness protocols. We thereby defined the signatures of neuronal activity resulting in tinnitus loudness decreases/increases within the same participants.
6.5. Auditory alpha power modulation for the individual patients.
The group results showed a high inter-individual variance for all investigated stimulation protocols, but also hinted at the potential of rTMS for treating tinnitus when it is applied in an individually optimized way (i.e. selecting protocol that increases auditory alpha power, see results section; see also Discussion indicating that this issue needs more definite confirmation). In order to address the clinically highly relevant question of the neuronal changes on an individual level we decided to add a single subject analysis. This was done to elucidate the pattern of how auditory alpha activity is modulated in the individual patients by the different stimulation protocols, in order to obtain information how the high variability within the protocols is made up. We therefore repeated the same analysis as described in the section on ‘Spectral power analyses derived from auditory cortex’, focussing on alpha power (as this was the most illuminating frequency band based on the group level results; see results section) however this time projecting the complex fourier spectra of the single trials onto our sources of interest. We then performed a single patient statistic by comparing the pre-post ratios of the single trials for each patient and TMS condition separately. Therefore, 5000 bootstrap replicates of the median were generated. We subsequently extracted the upper and lower quantiles corresponding to a probability of 5% and obtained the confidence intervals (CI) for each patient and stimulation form, respectively. This bootstrap procedure was in line with the one described above. Note that we here did not control for multiple comparisons so that the results should be interpreted on an explorative level only.
In a second step, we defined the most effective stimulation protocol in reducing tinnitus loudness according to the TIM (as already described for the group level in the section on ‘Signatures of auditory brain activity reducing tinnitus loudness’) for each patient separately and performed a single patient statistic quantifying the auditory alpha power modulation for the selected stimulation protocols. We further related the extent of the auditory alpha power modulation to the extent of the tinnitus loudness reduction (ranked from 1 to 10 with 1 reflecting the strongest loudness reduction). This procedure was repeated for the stimulation protocols that increased tinnitus loudness.
6.6. Signatures of whole brain activity reducing tinnitus loudness.
Although it was not the focus of the present study, we examined the signatures of oscillatory brain activity in non-auditory regions that are decisive for a strong decrease or increase in tinnitus loudness. We thus performed a whole brain analysis for the stimulation protocol (individually selected) that most effectively reduced or enhanced tinnitus loudness according to the TIM and analysed power modulations from pre to post-TMS in the frequency bands of interest (delta, theta, alpha, low gamma, high gamma). For this purpose, we performed Dynamic Imaging of Coherent Sources (DICS), introduced by Gross and colleagues [43]. This beamformer technique optimally estimates the power for a certain location while suppressing activity at all other locations. The headmodel and leadfield were taken from the prior ROI analysis. For each grid point, we constructed a spatial filter from the cross-spectral density matrix of the MEG signal (not ICA-cleaned) at the frequency of interest (delta, theta, alpha, low and high gamma) and the respective leadfield. Thereafter we applied the spatial filters to the Fourier-transformed ICA-cleaned data (multitaper analysis) for the frequency of interest and divided the values by an estimate of the spatially inhomogenous noise (obtained for each gridpoint on the basis of the smallest value of the covariance matrix) in order to normalise this across participants. Afterwards we interpolated the resulting activation volumes to the individual MRI of the patients and normalised them to a template MNI brain provided by the SPM2 toolbox (http://www.fil.ion.ucl.ac.uk/spm/software/spm2). For statistical analysis, we calculated (post-pre)/pre ratios for each voxel of the source solutions respectively and tested these relative values against zero by applying a voxel-wise t-statistic. To correct for multiple comparisons, we defined a minimum cluster size (minimum number of neighbouring voxels above a given threshold that are required for a significant cluster) with AlphaSim provided by the Afni Package (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim.pdf). We thereby preserved the main non-auditory regions (>770 voxels) that were modulated by an effective tinnitus loudness-reducing or enhancing TMS stimulation in the frequency bands of interest.
Results
1. Individual Tolerance of the TMS Stimulation
None of the patients showed serious side effects of rTMS apart from transient mild to moderate discomfort due to muscle contractions, involuntary movements of the jaw and cutaneous sensations during TMS stimulation. One patient experienced a mild headache after stimulation, which disappeared without medication after several hours. Another patient reported periods of complete absence of the tinnitus lasting for several minutes after 1 Hz rTMS. Three patients reported a worsening of their tinnitus after IAF stimulation lasting for several hours up to a few days…
Read the rest of the article here
